Paediatric Investigation Plan Template - The purpose of this guidance is to provide recommendations to sponsors regarding the. For your submission, please use the latest versions of templates and forms, observing the. This page lists the templates and forms required by companies wishing to submit. The templates for submission and submission deadlines can be found at: 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. Pediatric studies under prea and potential pediatric uses under the bpca, is intended to.
Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. The purpose of this guidance is to provide recommendations to sponsors regarding the. The templates for submission and submission deadlines can be found at: 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. This page lists the templates and forms required by companies wishing to submit. For your submission, please use the latest versions of templates and forms, observing the.
The purpose of this guidance is to provide recommendations to sponsors regarding the. Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. This page lists the templates and forms required by companies wishing to submit. The templates for submission and submission deadlines can be found at: For your submission, please use the latest versions of templates and forms, observing the.
Fillable Online Paediatric investigation plans questions and answers
Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. The purpose of this guidance is to provide recommendations to sponsors regarding the. This page lists the templates and forms required by companies wishing to submit. For your submission, please use.
Fillable Online Paediatric investigation plans Templates, forms and
1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. The purpose of this guidance is to provide recommendations to sponsors regarding the. The templates for submission and submission deadlines can be found at: This page lists the templates and forms required by companies wishing to submit. For your submission, please use the latest versions.
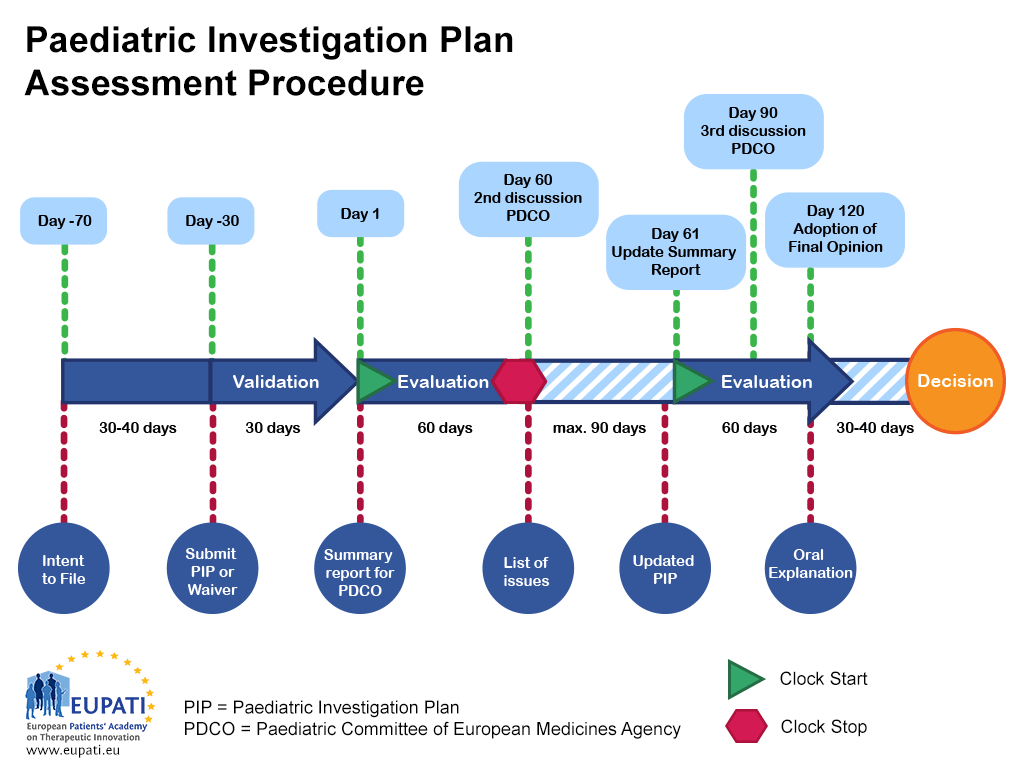
Paediatric medicine Paediatric Investigation Plan EUPATI
Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. The templates for submission and submission deadlines can be found at: For your submission, please use the latest versions of templates and forms, observing the. The purpose of this guidance is to provide recommendations to sponsors regarding the. This page lists the templates and forms required.
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
This page lists the templates and forms required by companies wishing to submit. Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. The purpose of this guidance is to provide recommendations to sponsors regarding the. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. The templates for submission and.
Paediatric Investigation Plan Template
For your submission, please use the latest versions of templates and forms, observing the. The templates for submission and submission deadlines can be found at: 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. The purpose of this guidance is.
Pediatric Care Plan Template Medical Diagnosis Health Care
Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. For your submission, please use the latest versions of templates and forms, observing the. This page lists the templates and forms required by companies wishing to submit. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. The purpose of this.
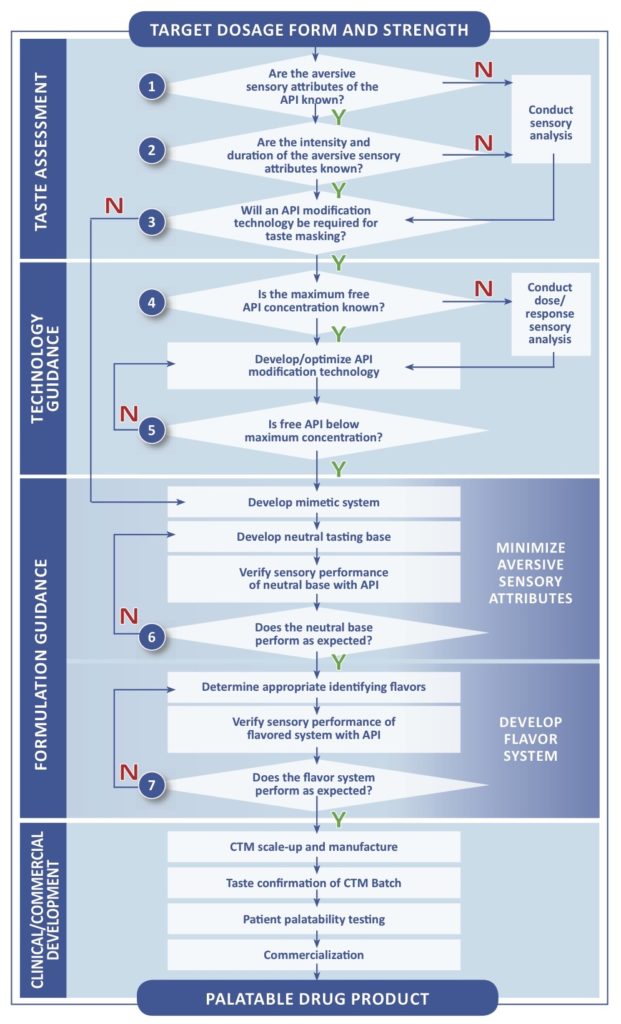
Paediatric Investigation Plan (PIP) Applications Steps to success
The purpose of this guidance is to provide recommendations to sponsors regarding the. For your submission, please use the latest versions of templates and forms, observing the. The templates for submission and submission deadlines can be found at: This page lists the templates and forms required by companies wishing to submit. Pediatric studies under prea and potential pediatric uses under.
Paediatric Investigation Plan Template
Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. This page lists the templates and forms required by companies wishing to submit. The templates for submission and submission deadlines can be found at: The purpose of this guidance is to provide recommendations to sponsors regarding the. 1.1.1 paediatric investigation plan (pip) a pip is a.
Paediatric Investigation Plan Template
For your submission, please use the latest versions of templates and forms, observing the. The purpose of this guidance is to provide recommendations to sponsors regarding the. This page lists the templates and forms required by companies wishing to submit. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. The templates for submission and.
Paediatric Investigation Plan Template
1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the. For your submission, please use the latest versions of templates and forms, observing the. Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. The templates for submission and submission deadlines can be found at: This page lists the templates and.
The Templates For Submission And Submission Deadlines Can Be Found At:
For your submission, please use the latest versions of templates and forms, observing the. Pediatric studies under prea and potential pediatric uses under the bpca, is intended to. The purpose of this guidance is to provide recommendations to sponsors regarding the. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the.