Monitoring Plan Template For Clinical Trials - Niams has guidelines and templates to help. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Clinical monitoring and data management plan (cmp/dmp) checklist. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Throughout the template there are suggested. This template is a suggested format for a monitoring plan developed by tb survey teams. Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library.
Clinical monitoring and data management plan (cmp/dmp) checklist. Throughout the template there are suggested. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. Niams has guidelines and templates to help. Welcome to global health trials' tools and templates library. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Please note that this page has been updated for 2015 following a quality check. This template is a suggested format for a monitoring plan developed by tb survey teams.
Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. Clinical monitoring and data management plan (cmp/dmp) checklist. Welcome to global health trials' tools and templates library. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. This template is a suggested format for a monitoring plan developed by tb survey teams. Throughout the template there are suggested. Please note that this page has been updated for 2015 following a quality check. Niams has guidelines and templates to help.
Monitoring Report Template Clinical Trials
Throughout the template there are suggested. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Welcome to global health trials' tools and templates library. Niams has guidelines and templates to help. This template is a suggested format for a monitoring plan developed by tb survey teams.
A Centralized Monitoring Approach Using Excel For The within Monitoring
Clinical monitoring and data management plan (cmp/dmp) checklist. Welcome to global health trials' tools and templates library. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Throughout the template there are suggested. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the.
Monitoring Report Template Clinical Trials
Please note that this page has been updated for 2015 following a quality check. Clinical monitoring and data management plan (cmp/dmp) checklist. Throughout the template there are suggested. Welcome to global health trials' tools and templates library. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study.
Medical Monitoring Plan Template
Welcome to global health trials' tools and templates library. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Niams has guidelines and templates to help. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Throughout the template there are suggested.
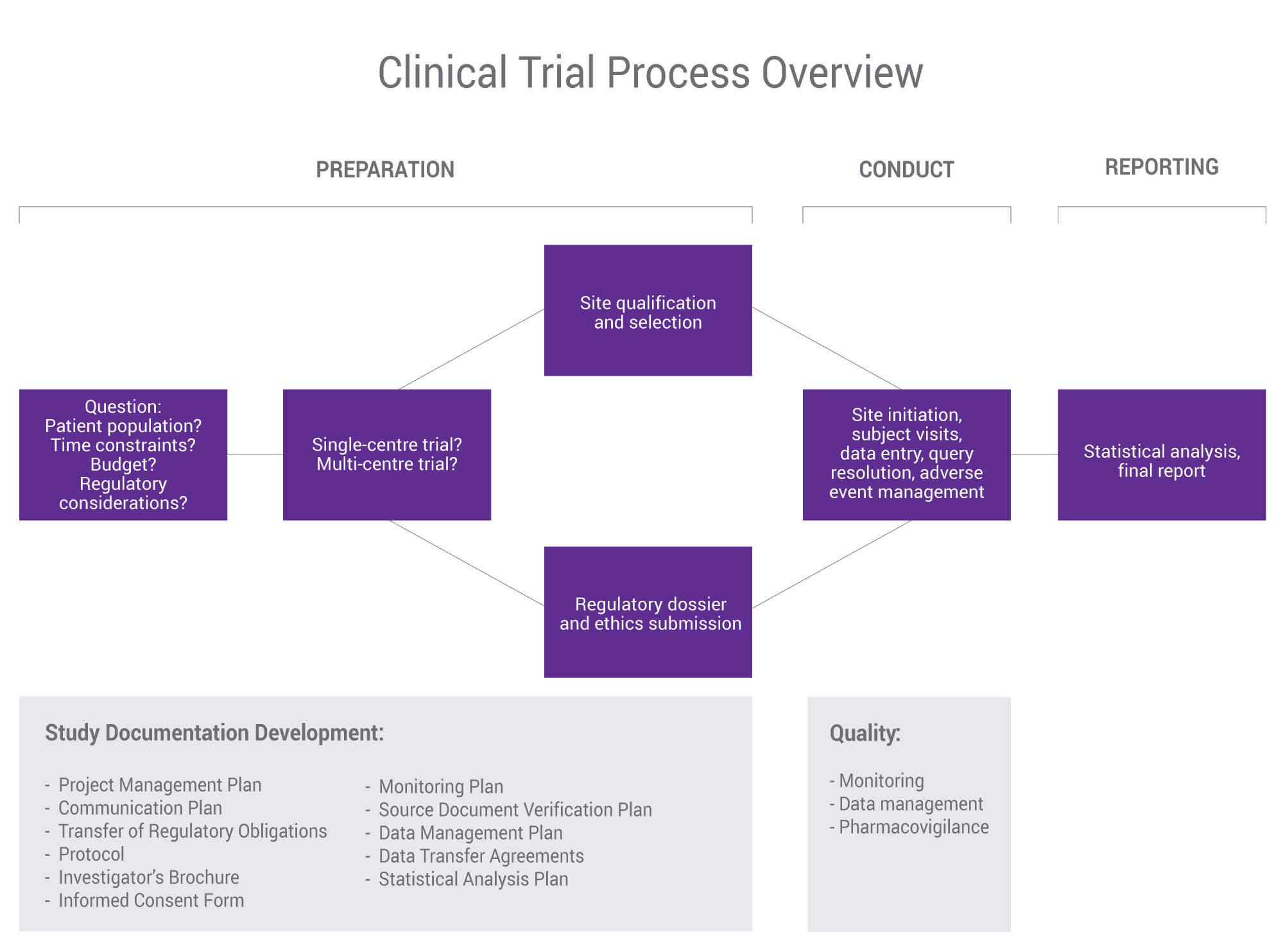
Clinical Trial Project Management Plan Template
How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. This template is a suggested format for a monitoring plan developed by tb survey teams. Niams.
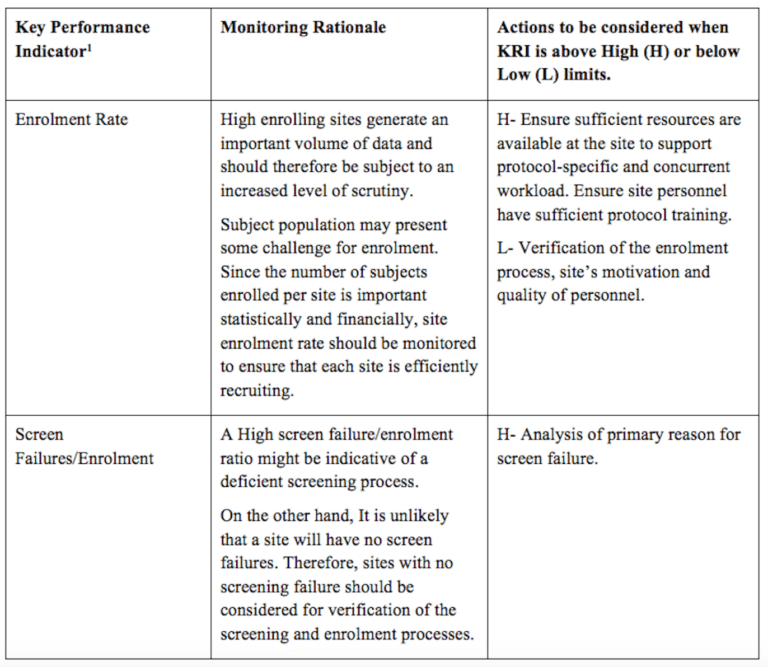
3.8. Template for monitoring plan
Niams has guidelines and templates to help. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. Clinical monitoring and data management plan (cmp/dmp) checklist. Welcome.
Monitoring Plan Template For Clinical Trials
How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. This template is a suggested format for a monitoring plan developed by tb survey teams. Niams has guidelines and templates to help. Please note that this page has been updated for 2015 following a quality check. Clinical monitoring and data.
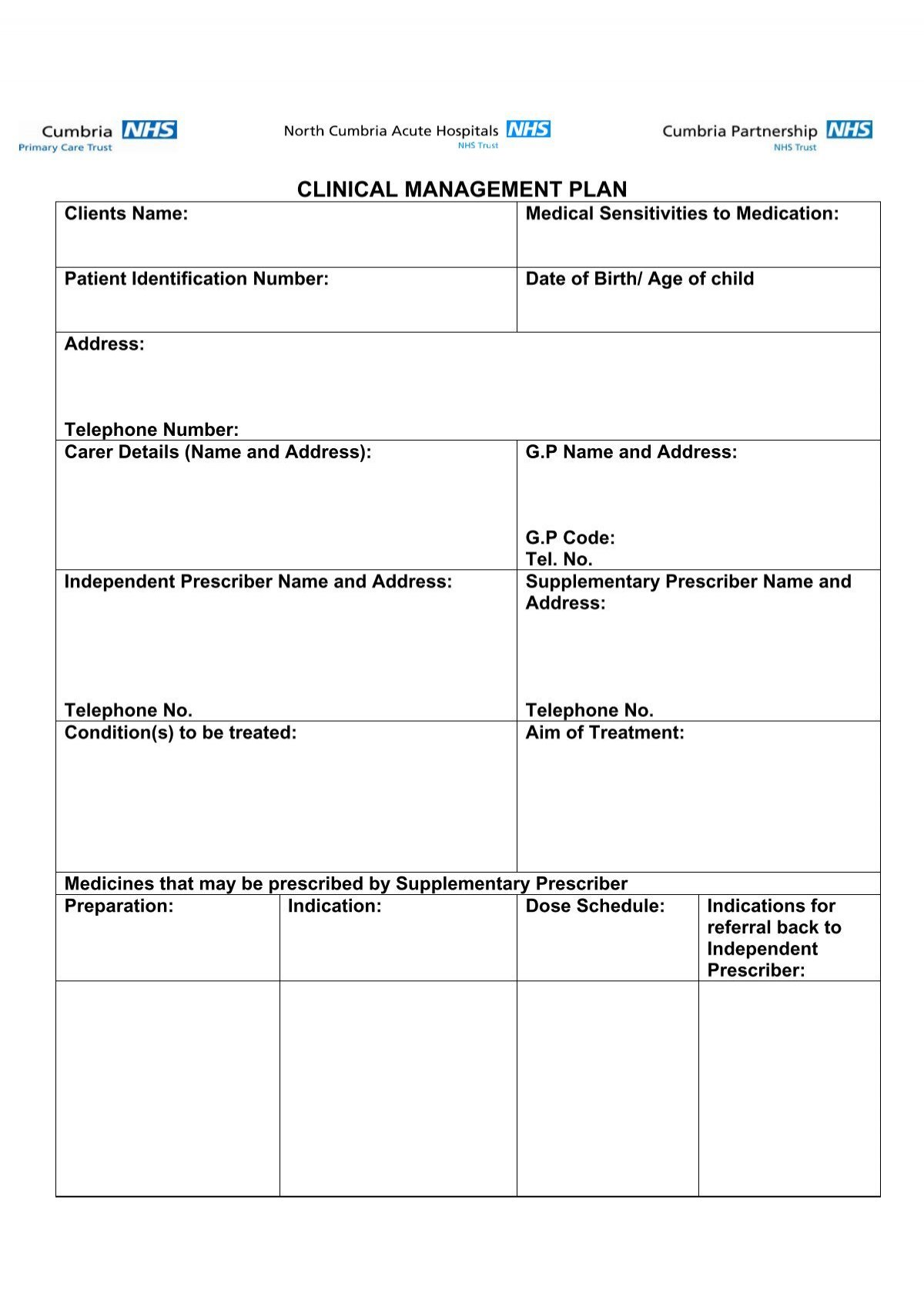
Clinical management plan template NHS Cumbria
Niams has guidelines and templates to help. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Throughout the template there are suggested. This template is.
Management Plans Al Trial Project Plan Example Template with regard to
How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality check..
Clinical Trial Monitoring Plan Template
This template is a suggested format for a monitoring plan developed by tb survey teams. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Welcome to global health trials' tools and templates library. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or.
Throughout The Template There Are Suggested.
Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Niams has guidelines and templates to help.
This Template Is A Suggested Format For A Monitoring Plan Developed By Tb Survey Teams.
Please note that this page has been updated for 2015 following a quality check. Clinical monitoring and data management plan (cmp/dmp) checklist. Welcome to global health trials' tools and templates library.