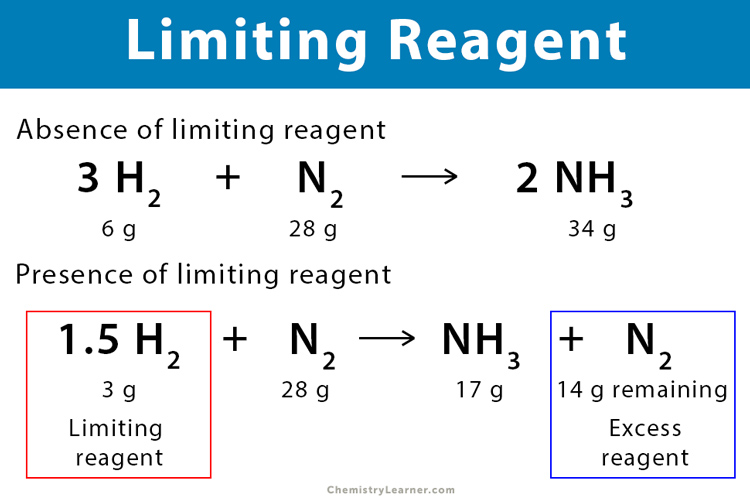
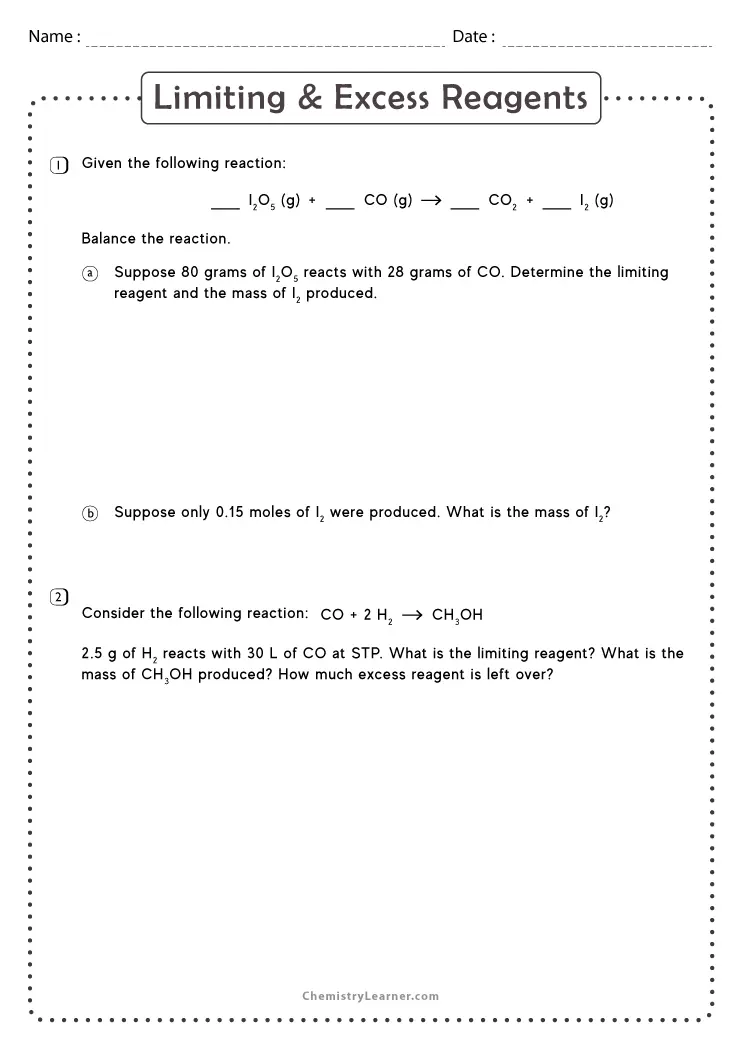
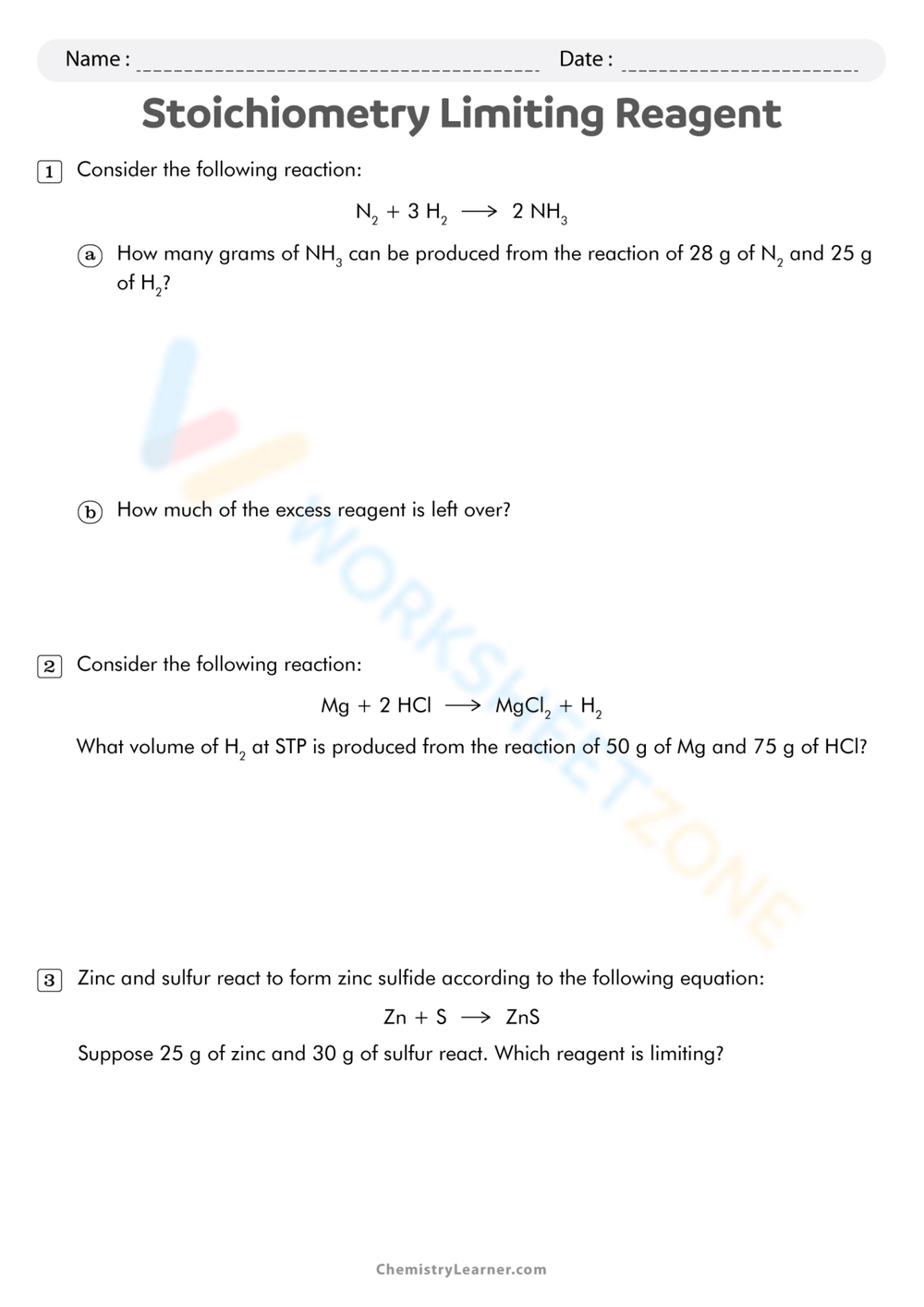
Limiting And Excess Reactants Worksheet With Answers - A student places 2.36 moles of acetic acid. Hydrogen is limiting and carbon monoxide is in excess. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? B) how many grams of zns will be formed? C) how many grams of the excess reactant will remain after. (b) how many moles of carbon dioxide gas (co2) will form? When 3.22 moles of al reacts with. Students must determine which reactant is in excess. A) which chemical is the limiting reactant? (a) identify the limiting reactant.
Students must determine which reactant is in excess. A) which chemical is the limiting reactant? B) how many grams of zns will be formed? C) how many grams of the excess reactant will remain after. When 3.22 moles of al reacts with. Students must determine which reactant is in excess. Hydrogen is limiting and carbon monoxide is in excess. A student places 2.36 moles of acetic acid. (b) how many moles of carbon dioxide gas (co2) will form? How many moles of nh3 can be produced from the reaction of 28 g of n2 ?
When 3.22 moles of al reacts with. (a) identify the limiting reactant. Limiting reactant practice ws 1. Determine how much product each reactant can produce. Students must determine which reactant is in excess. C) how many grams of the excess reactant will remain after. Worksheet on excess and limiting reactants/reagents. A student places 2.36 moles of acetic acid. Hydrogen is limiting and carbon monoxide is in excess. Worksheet on excess and limiting reactants/reagents.
How To Determine Limiting And Excess Reactant
Students must determine which reactant is in excess. Worksheet on excess and limiting reactants/reagents. (b) how many moles of carbon dioxide gas (co2) will form? How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reactant practice ws 1.
SOLUTION 24 limiting and excess reactants s Studypool
Students must determine which reactant is in excess. When 3.22 moles of al reacts with. Determine how much product each reactant can produce. (b) how many moles of carbon dioxide gas (co2) will form? 2 al + 6 hr → 2 alr 3 + 3 h 2 a.
Limiting Reagent Problems And Answers Limiting Reagent (reac
Worksheet on excess and limiting reactants/reagents. B) how many grams of zns will be formed? When 3.22 moles of al reacts with. (b) how many moles of carbon dioxide gas (co2) will form? A student places 2.36 moles of acetic acid.
Limiting And Excess Reactants Worksheet Key
C) how many grams of the excess reactant will remain after. Determine how much product each reactant can produce. Students must determine which reactant is in excess. (b) how many moles of carbon dioxide gas (co2) will form? When 3.22 moles of al reacts with.
Limexcess reagants wksht and answers Practice Problems Limiting
Determine how much product each reactant can produce. (a) identify the limiting reactant. Students must determine which reactant is in excess. Limiting reactant practice ws 1. B) how many grams of zns will be formed?
How to Solve Limiting and Excess Reactants Worksheet with PDF Answers
Students must determine which reactant is in excess. 2 al + 6 hr → 2 alr 3 + 3 h 2 a. Worksheet on excess and limiting reactants/reagents. B) how many grams of zns will be formed? Students must determine which reactant is in excess.
Limiting And Excess Reactants Worksheets With Answers
Worksheet on excess and limiting reactants/reagents. A) which chemical is the limiting reactant? Students must determine which reactant is in excess. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Students must determine which reactant is in excess.
Limiting And Excess Reagent Worksheet Answer Formula Calcula
(a) identify the limiting reactant. Determine how much product each reactant can produce. Students must determine which reactant is in excess. B) how many grams of zns will be formed? C) how many grams of the excess reactant will remain after.
Limiting And Excess Reactants Worksheets With Answers
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? 2 al + 6 hr → 2 alr 3 + 3 h 2 a. When 3.22 moles of al reacts with. Limiting reactant practice ws 1. A) which chemical is the limiting reactant?
Free Printable Limiting Reactant and Percent Yield Worksheets
Students must determine which reactant is in excess. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reactant practice ws 1. (b) how many moles of carbon dioxide gas (co2) will form? When 3.22 moles of al reacts with.
A) Which Chemical Is The Limiting Reactant?
Limiting reactant practice ws 1. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? 2 al + 6 hr → 2 alr 3 + 3 h 2 a. Students must determine which reactant is in excess.
(A) Identify The Limiting Reactant.
Worksheet on excess and limiting reactants/reagents. (b) how many moles of carbon dioxide gas (co2) will form? Hydrogen is limiting and carbon monoxide is in excess. When 3.22 moles of al reacts with.
Worksheet On Excess And Limiting Reactants/Reagents.
Students must determine which reactant is in excess. A student places 2.36 moles of acetic acid. B) how many grams of zns will be formed? Determine how much product each reactant can produce.